New product launch | Alipore®TAHF PTFE material sterilization grade cartridge filter

Recently, Alioth Alipore®TAHF polytetrafluoroethylene (PTFE) material sterilization grade cartridge filter elements (5 inches, 10 inches, 20 inches, 30 inches) are officially on the market to protect the safe production of drugs by pharmaceutical enterprises.
Alipore®TAHF sterilization grade cartridge filter element
gas, non-aqueous phase sterilization filtration
Alioth Alipore® TAHF series is composed of hydrophobic and chemically inert PTFE (polytetrafluoroethylene) filter membrane. This product can efficient retain microorganism or particles in wet or humid gas through stringent liquid bacteria retention verification. High-strength, resistant to multiple steam sterilization in place. It is suitable for gases sterile filtration and organic solvents filtration with strict quality requirements in pharmaceutical applications.

Product Properties
- Natural hydrophobic material
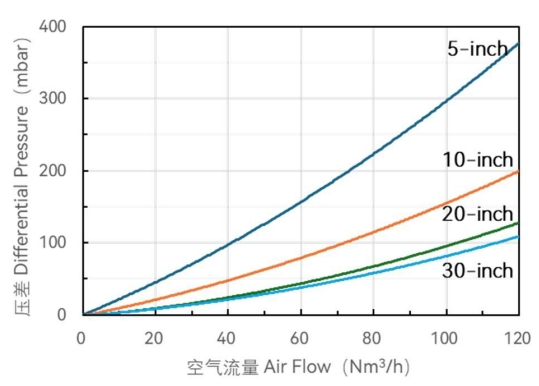
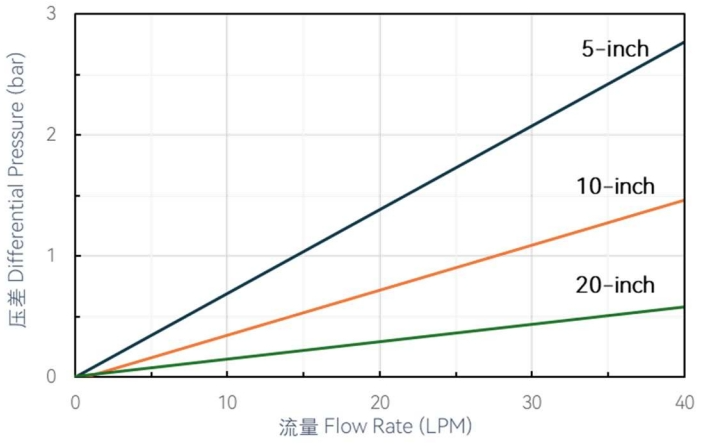
- High gas flux and low-pressure difference
- Reliable bacterial retention and particles removal ability
- Excellent chemical compatibility
Typical Applications
- Sterile filtration of N2、O2、CO2 and compressed air
- Breathing apparatus such as fermentor and liquid storage tanks
- Sterile filtration of freeze dryer and autoclaving equipment of exhaust gases
- Sterile filtration of most organic solvents
Product Characteristics
| Product specification | 5inch | 10inch | 20inch | 30inch | |
| Dimensions | Diameter Maximum Length |
69 mm 150 mm |
69 mm 320 mm |
69 mm 560 mm |
69 mm 800 mm |
| Filtration Area | 0.35 m2 | 0.7 m2 | 1.4 m2 | 2.1 m2 | |
| Pore Size | 0.2 μm | ||||
| Structural Materials | Filter membrane: Hydrophobic Polytetrafluoroethylene (PTFE) Supporting layer: Polyethylene O-rings: Silicone Liner / cartridge body: Polyethylene End caps: Polyethylene / Polycarbonate |
||||
| Bacterial Retention | >107 cfu/cm2 B. diminuta ( ATCC® 19146TM) | ||||
| Maximum Tolerated Differential | Forward: 5.0 bar(72.5 psi)@ 25°C / 3.0 bar(43.5 psi)@ 80°C Reverse: 2.0 bar(29.0 psi)@ 25℃ |
||||
| Bubble Point | ≥1200 mbar(17.4 psi)(wetted with 60% IPA, 20℃, compressed air) | ||||
| Diffusion | Through a 60% IPA wet membrane at 1040 mbar (15 psi) (20℃, compressed air ) | ||||
| ≤6.5 mL/min | ≤ 13 mL/min | ≤ 26 mL/min | ≤ 39 mL/min | ||
| Water Intrusion | at 2500 mbar (36 psi) (20℃, compressed air ) | ||||
| ≤ 0.25 mL/min | ≤ 0.5 mL/min | ≤ 1.0 mL/min | ≤ 1.5 mL/min | ||
| Sterilization | Steam in place: 135℃, 60 min, 25 cycles Autoclave: 135℃, 60 min, 25 cycles |
||||
| Biological Safety | All the construction components of this filter comply with the requirements for biological safety of Class VI plastics in current USP<88> | ||||
| Indirect Food Additive | The primary construction components of this filter comply with the requirements for food contact materials as stipulated in EU 1935 / 2004 / EC and FDA 21 CFR 177-182 |
||||
| Cleanliness | Cleanliness meets the requirements for nonfiber releasing filter as specified inFDA21 CFR211.72 and 210.3 (b) (5) (6), and the level of insoluble particles in rinsing liquid meets the requirements of USP< 788> | ||||
| Endotoxin | The endotoxin level of cartridge rinsing liquid is <0.25 EU/mL as indicated by gel method, which complies with the requirements of USP<85> | ||||
| TOC / Conductivity | Filtrate of this filter, TOC<0.5 mg/L, Conductivity<1.3 μS/cm | ||||
| Integrity | 100% has passed the integrity test | ||||
| Manufacturing Environment | Manufactured in a Class 100,000 clean workshop | ||||
Flow characteristics
Sample Application Channel
For more product information, please scan the two-dimensional code below to apply for the product manual:

If you need to apply for sample testing, please scan the following two-dimensional code:


