Single-layer Cartridge Filter(MCE 0.2μm)
Product Introduction
Alioth Aligard® MHF Single-layer Cartridge Filter is made from mixed cellulose ester. It has a nominal filtration accuracy of 0.2 μm, a sturdy structure and stable material. It can efficiently retain pollutants, effectively remove particles and colloidal contaminants, block no important active ingredients, protect downstream filtration equipment, and prevent premature clogging.
Key Features and Benefits
- Fast flow rate
- High capacity
- No fiber release
- Can withstand multiple cycles of high-temperature sterilization
Typical Applications
- Pre-filtration of culture media and buffer solutions
- Pre-filtration of LVP/SVP
- Pre-filtration of blood products
- Pre-filtration for column protection, etc.
Typical Flow Characteristics
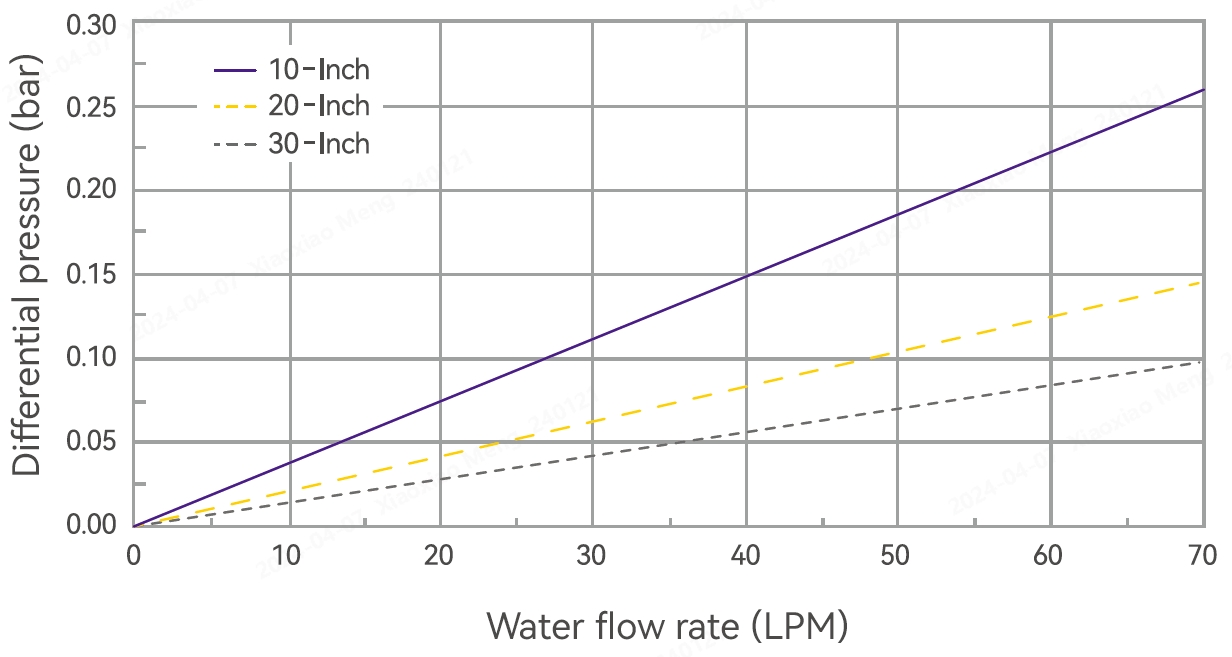
Product Specifications
| 10 inch | 20 inch | 30 inch | |
|
Dimensions Diameter |
69 mm |
69 mm |
69 mm |
| Filtration Area | 0.68 m2 | 1.32 m2 | 1.98 m2 |
| Pore Size | 0.2 μm | ||
| Materials of Construction |
Filter membrane:Mixed Cellulose Ester Supporting Layer: Polypropylene (PP) Liner / Cartridge body: Polypropylene (PP) |
||
| Maximum Differential Pressure |
Forward: 5 bar ( 72.5 psi ) @ 25℃ / 1.5 bar ( 21.75 psi ) @ 80℃ / Reverse: 2 bar ( 29 psi ) @ 25℃ | ||
| Sterilization Parameters | 121℃, 30 minutes, 5 cycles of high-pressure sterilizations or 5 cycles of sterilization in place | ||
| Extractable | Water: ~20 mg / 10 inch Ethanol: water ( 50:50 ): ~55 mg / 10 inch |
||
| Biological Safety | All the construction components of this cartridge comply with the requirements for biological safety of Class VI plastics in current USP < 88 > | ||
| Indirect Food Additive | The primary construction components of this cartridge comply with the reguirements for food contact materials as stipulated in EU 1935 / 2004 / EC and FDA 21 CFR 177-182 | ||
| Cleanliness | Cleanliness meets the requirements for nonfiber releasing filter as specified in FDA 21 CFR 211.72 and 210.3 (b) (5) (6), and the level of insoluble particles in rinsing liquid meets the requirements of USP < 788 > |
||
| Endotoxin | The endotoxin level of cartridge rinsing liquid is < 0.25 EU/mL as indicated by gel method, which complies with the requirements of USP < 85 > | ||
| Integrity | Each filter has passed the integrity test | ||
| TOC / Conductivity | TOC < 0.5 mg/L, Conductivity < 1.3 μS/cm | ||
| Manufacturing environment | Manufactured in conformance with cGMP | ||
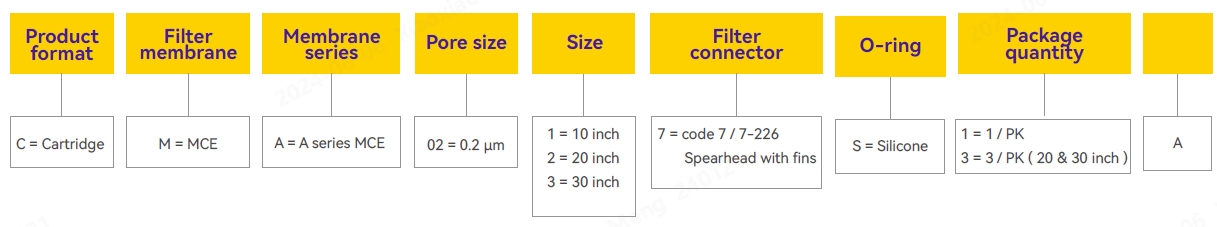
Ordering Information
For further information, please contact us and Alioth technical team will provide a comprehensive service for your filtration process.


