Manual Ultrafiltration System
Product Introduction
Alioth Manual Ultrafiltration System, with its modular design and flexible combination options, supports the use of filters or hollow fibers of different areas, thus realizing various filtration requirements ranging from a minimum of 0.1 m2 to a maximum of 20 m2, or even a larger area. It is operated manually, providing convenient control. Both desktopand ground-based versions are available to meet different site needs. The overall system design is compact and space-saving. By displaying pressure and manually adjusting flow rate, the system can control TMP and Delta P effectively. It has flexible design, versatile functions, and diverse applications, thus suitable for various stages and needs in pharmaceutical industry, such as laboratory-scale process development, pilot-scale drug production, and GMP-level drug production. Customers can configure different functional modules, such as flow rate, temperature, pH, conductivity, and UV, according to their own process requirements. It has a sanitary design and provides complete IQ / OQ verification service and document support. It can be applied in various pharmaceutical production processes, including but not limited to concentration/diafiltration, microfiltration, and unidirectional tangential flow filtration.
Product Properties
- Compatible with different types of consumables, and flat filters or hollow fibers of different areas
- Compatible with mainstream brands of filters and hollow fibers in the market
- Sanitary design, with main liquid-contacting materials being 316 L stainless steel and FDA-compliant non-metal materials such as
- EPDM / PTFE/TPE commonly used in pharmaceutical industry
- Resistant to acid and alkali corrosion, liquid-contacting surfaces can be selected with Ra < 0.4 μm EP or Ra < 0.6 μm MP as needed
- The holder adopts standard TC connector, allowing free adaptation to the system
- Compact and rational system design, resulting in a small footprint
- Simple system configuration and convenient operation
- Can adapt to a filtration area of approximately 0.1-20 m2
- Pump overpressure protection, mechanical interlocking, safe and reliable operation
- Reliable quality, traceable process, accessible liquid-contacting material certificate and cleanliness certificate
- High-quality service, and accessible FAT / SAT / IQ / OQ service packages that can be purchased according to needs
Product Applications
- Vaccine purification
- Virus purification
- Blood product purification
- Monoclonal antibody purification
- Recombinant protein purification
- Endotoxin removal
PID Flowchart

Product Characteristics
| Membrane area | Common filter series: 0.1-0.5 m2 |
Common filter series: 0.5-2.5 m2 |
Common filter series: 5-10 m2 |
Common filter series: 10-20 m2 |
| Pump | Optional diaphragm pump or peristaltic pump | |||
| Operation mode | Manual control of TMP, manual control of valves, manual recording of data | |||
| Power supply | 220V / 50Hz ( P + N + PE ) or 380 V / 50 Hz (3 P + N + PE) | |||
| System pressure resistance | Maximum 6 bar | |||
| Operating temperature | SS 316 L; Polymer; EPDM; Silicone; TPE | |||
| Pipeline material | 4 - 40°C recommended for plastic material part; 4 - 60°C recommended for SS 316 L part | |||
| Sanitary connection method | Sanitary chuck | |||
| Liquid-contacting surface finish |
Optional: Ra < 0.4 μm EP or Ra < 0.6 μm MP | |||
※For ultrafiltration system with a larger area or other customization requirements, please contact local sales and technical personnel
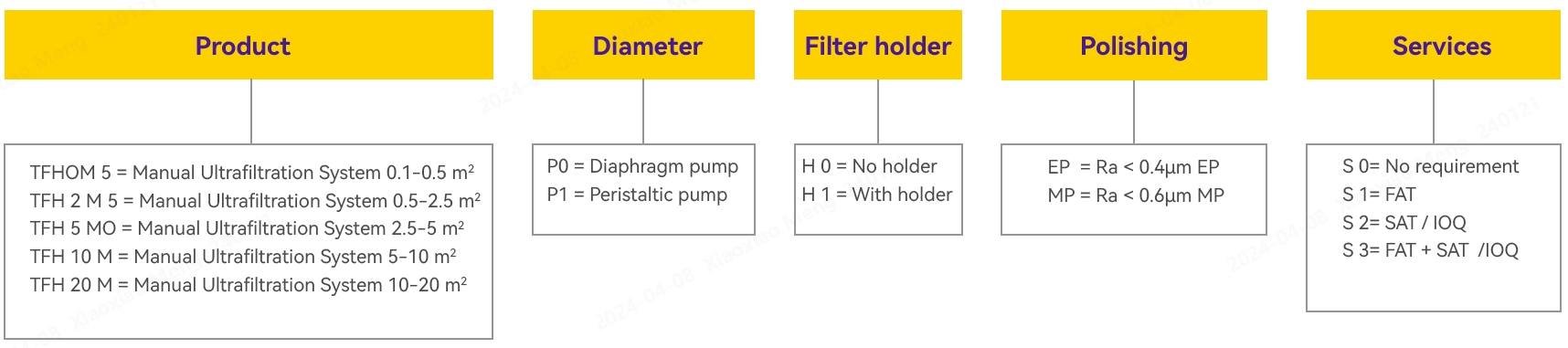
Ordering Information
For further information, please contact us and Alioth technical team will provide a comprehensive service for your filtration process.


