Fully Automatic Large-scale Ultrafiltration System
Product Introduction
Alioth Fully Automatic Ultrafiltration System adopts modular design, and can achieve tangential flow filtration for different processes by matching with different specifications of filter holders or hollow fibers. The system is compact, practical, and powerful, featuring formula editing and one-click fully automatic operation. This system is highly automated and meets the requirements of domestic and international GMP production regulations. It is the best choice for pharmaceutical GMP production, especially suitable for production needs involving strict regulations such as FDA certification and EUGMP certification.
Product Properties
- All system components are selected from leading brands in the industry, adopt sanitary design and comply with ASMEBPE requirements
- Compatible with tangential flow filters and hollow fibers of different areas, ranging from 0.02 m2 to 200 m2
- Compatible with ultrafiltration filters and hollow fiber column consumables
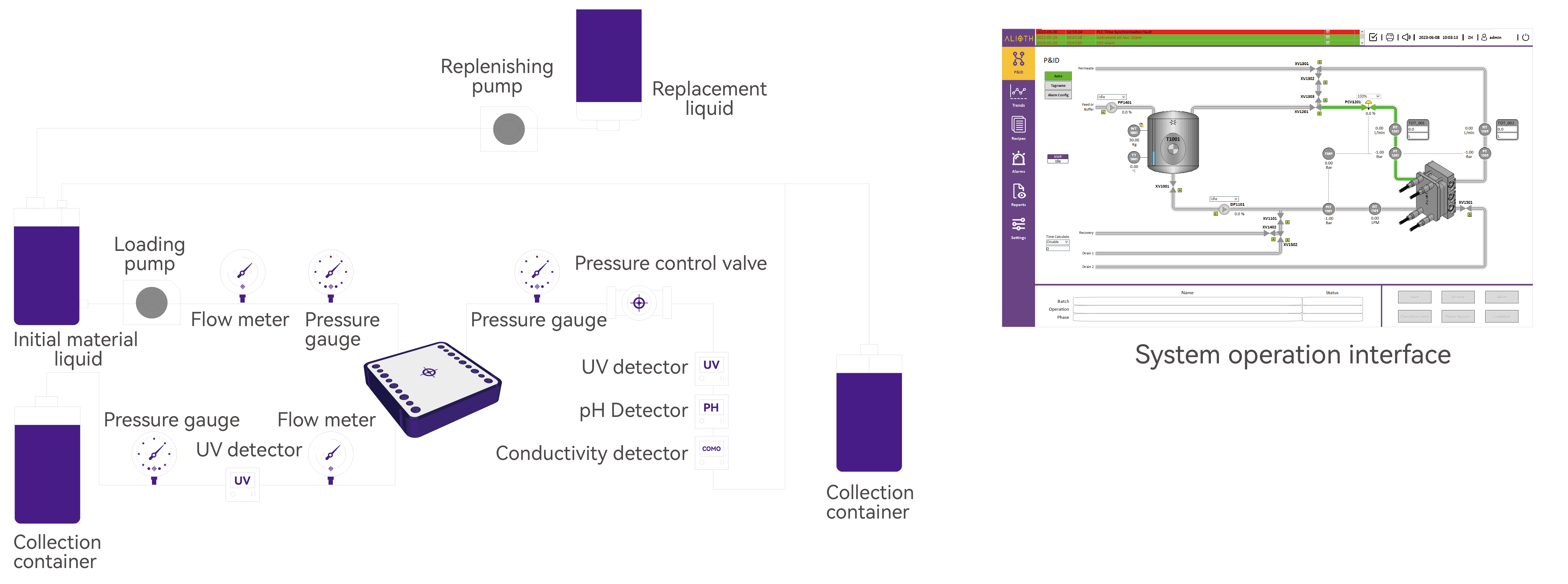
- Configurable PLC, weighing unit, pneumatic valves, circulation pump, feeding pump, flow meter, pressure gauge, pH, conductivity and UV, etc.; feedback control of TMP and Delta P; automated processes such as concentration, equal volume washing and filtration, overconcentration, and CIP
- Configurable process tanks of different volumes and requirements; equipped with sight glass, spraying ball, magnetic stirring, sampling valve, and heat insulation jacket; precise control of jacket refrigerant opening through regulating valve and feedback regulation of product temperature
- Compatible with disposable liquid storage and dispensing systems, flexible and convenient
- Can be equipped with online detection units for flow, pressure, temperature, conductivity, pH and UV, etc.; through real-time recording and pooling to generate full-process parameter trends and PDF reports
- Configurable online integrity testing module for filter, automatically perform the integrity testing for multiple filters and generate electronic batch reports, without the need for external integrity testing instruments
- Configurable CIP, SIP, water flux detection, and filter integrity testing functions, and independently generate electronic batch reports
- One-click fully automatic operation with full-process feedback control
- Bilingual operation interface in both Chinese and English
- Equipped with batch management functionality
- Equipped with grading alarm functionality
- Equipped with four-tier authorization management functionality
- Compliant with data integrity regulations
- Compliant with the regulations for electronic records and electronic signatures
- Compliant with audit traceability regulations
- Can communicate and docking with third-party SCADA platforms.
- Can remotely interconnect with and control third-party DCS platforms such as DeltaV, PCS7, Plant Pax
- System design can be tailored to customer process requirements, plant layout, and utility system point requirements through scientifical and reasonable three-dimensional design
- Multiple types of customization available according to the requirements of customer
Product Applications
- Vaccine purification
- Monoclonal antibody purification
- Blood product purification
- Purification of novel therapeutic drugs
PID Flowchart
Product Characteristics
| Product specification | 2.5-5m2 | 5-10m2 | 10-20m2 | 20m2+++ |
| Product code | TFA5M0 | TFA10M | TFA20M | CUSTOM |
| Automatic control level | Automatic control of TMP, automatic control of valves, and automatic recording of data | |||
| Operating pressure | Maximum 5 bar | |||
| System pressure resistance | Maximum 6 bar | |||
| Pipeline fittings | SS 316 L Ra ≤ 0.6 μm (0.4 μm optional) | |||
| Sanitary connection | Tri Holder | |||
| Manual valve | PTFE or EDPM | |||
| Pressure measurement | 0-6 bar, ± 0.1 | |||
| Flow detection | Permeating end: mass flow meter; reflux end: electromagnetic flow meter | |||
| Electrical components | SIEMENS S 7 | |||
| Operation mode | Software automatic recording and analysis |
Operating system | Win 10 | |
| Minimum circulation volume and residual volume |
2.5-5 m2, minimum circulation volume < 2 L and residual volume < 10 mL 5-10 m2, minimum circulation volume < 5 L and residual volume < 10 mL 10-20 m2, minimum circulation volume < 10 L and residual volume < 10 mL For customized equipment, theoretical values are calculated based on actual 3D design |
|||
| Supported languages | Bilingual (Chinese and English), support Chinese input method | |||
| Materials of liquid-contact parts |
316 L, Polymer (USP class VI or FDA), EPDM, Santoprene elastomer, medical, Grade epoxy, silicone etc | |||
※Fully automatic systems have various requirements, flexible configurations, and are relatively complex. It is recommended to contact local sales and technical personnel for consultation as soon as possible
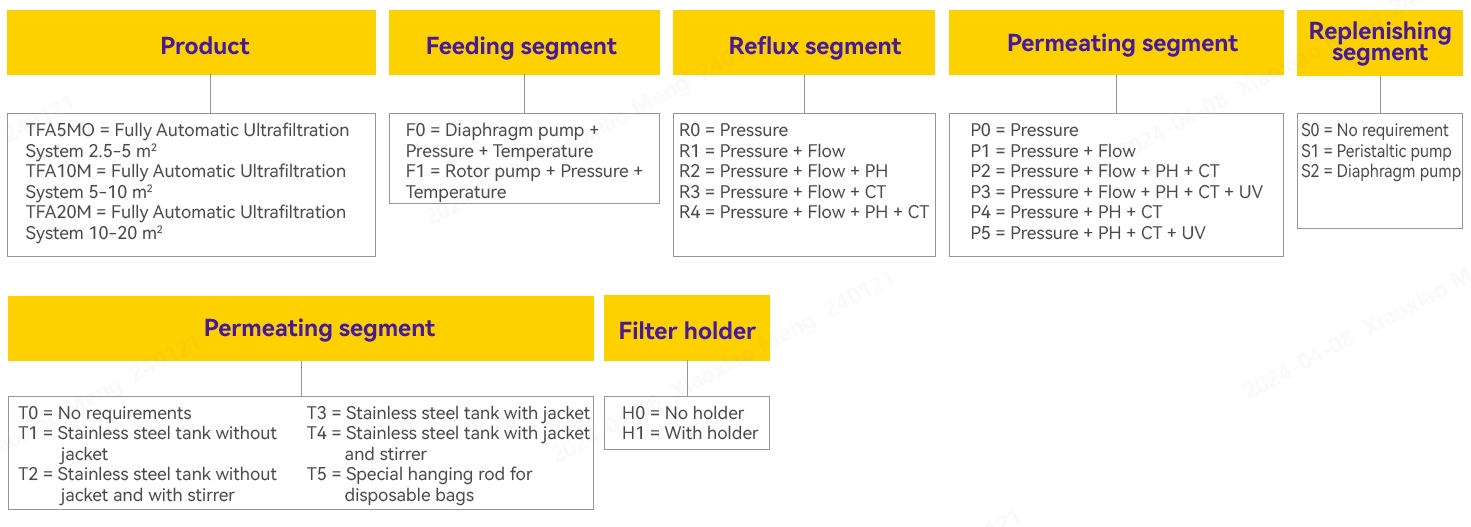
Ordering Information
For further information, please contact us, Alioth technical team will provide a comprehensive service for your filtration process.


