Sterilizing-Grade Cartridge Filter(PTFE 0.2μm)
Product Introduction
The Alioth Alipore® TAHF series features a hydrophobic and chemically inert PTFE (polytetrafluoroethylene) filter membrane. This product efficiently retains microorganisms and particles in wet or humid gas through stringent liquid bacteria retention verification. It offers high strength and resistance to multiple steam sterilizations in place. The TAHF series is suitable for sterile gas filtration and organic solvent filtration with strict quality requirements in pharmaceutical applications.
Key Features and Benefits
- Natural hydrophobic material
- High gas flux and low-pressure drop
- Reliable bacterial retention and particles removal ability
- Excellent chemical compatibility
Typical Applications
- Sterile gas filtration
- VentSterile filtration of most organic solvents
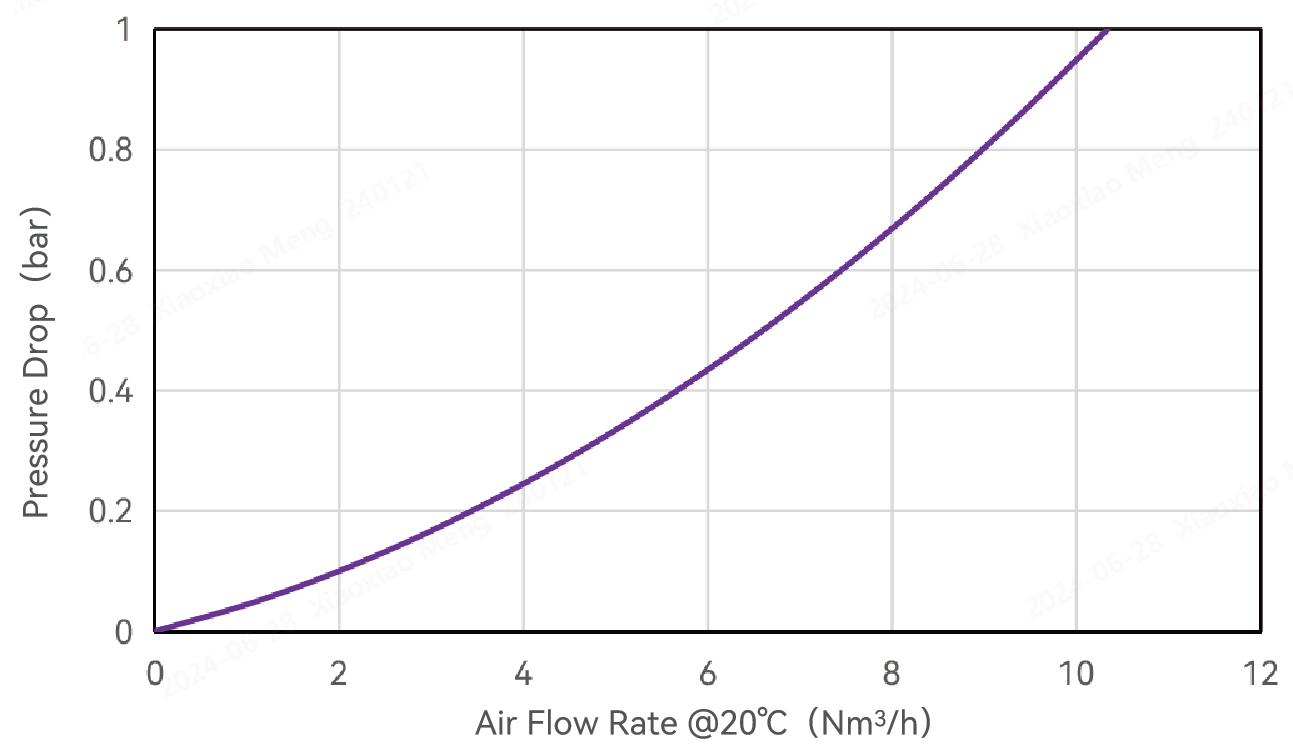
Typical Flow Characteristics
Air Flow Rate and Pressure Drop -Alipore® TAHF 0.2μm Cartridge Filters

Air Flow Rate and Pressure Drop -Alipore® TAHF 0.2μm Alicap L300 Capsule Filters
(KFBTA02S2B6S1A)
Product Specifications—Cartridge Filter
| 5inch | 10inch | 20inch | 30inch | ||
|
Dimensions
Diameter |
69 mm |
69 mm |
69 mm |
69 mm |
|
| Filtration Area | 0.35 m2 | 0.7 m2 | 1.4 m2 | 2.1 m2 | |
| Pore Size | 0.2 μm | ||||
| Materials of Construction |
Filter membrane: Polytetrafluoroethylene (PTFE) Supporting Layer: Polypropylene (PP) Liner / Cartridge body: Polypropylene (PP) |
||||
| Bacterial Retention | >107 cfu/cm2 B. diminuta ( ATCC® 19146TM) | ||||
| Maximum
Differential Differential |
Forward: 5.0 bar(72.5 psi)@ 25°C / 3.0 bar(43.5 psi)@ 80°C Reverse: 2.0 bar(29.0 psi)@ 25℃ |
||||
| Bubble Point | ≥1200 mbar(17.4 psi)(wetted with 60% IPA, 20℃, compressed air) | ||||
| Diffusion | Through a 60% IPA wet membrane at 1040 mbar (15 psi) (20℃, compressed air ) | ||||
| ≤6.5 mL/min | ≤ 13 mL/min | ≤ 26 mL/min | ≤ 39 mL/min | ||
| Water Intrusion | at 2500 mbar (36 psi) (20℃, compressed air ) | ||||
| ≤ 0.25 mL/min | ≤ 0.5 mL/min | ≤ 1.0 mL/min | ≤ 1.5 mL/min | ||
| Sterilization | Steam in place: 135℃, 60 min, 25 cycles Autoclave: 135℃, 60 min, 25 cycles |
||||
| Biological Safety | All the construction components of this filter comply with the requirements for biological safety of Class VI plastics in current USP<88> | ||||
| Indirect Food Additive | The primary construction components of this filter comply with the requirements for food contact materials as stipulated in EU 1935 / 2004 / EC and FDA 21 CFR 177-182 |
||||
| Cleanliness | Cleanliness meets the requirements for nonfiber releasing filter as specified inFDA21 CFR211.72 and 210.3 (b) (5) (6), and the level of insoluble particles in rinsing liquid meets the requirements of USP< 788> | ||||
| Endotoxin | The endotoxin level of cartridge rinsing liquid is <0.25 EU/mL as indicated by gel method, which complies with the requirements of USP<85> | ||||
| Integrity | 100% has passed the integrity test | ||||
| Manufacturing Environment |
Manufactured in conformance with cGMP |
||||
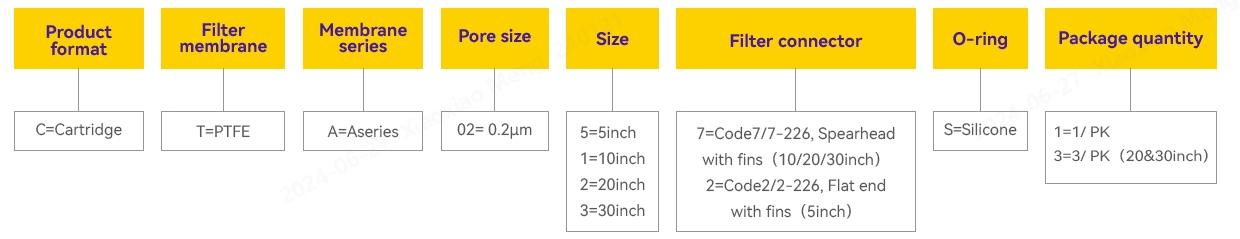
Ordering Information
For further information, please contact us and Alioth technical team will provide a comprehensive service for your filtration process.


