Depth Filters
Alioth Alidep® Depth Filter is mainly used for the clarification step in pharmaceutical process, and its disposable design provides greater flexibility for large-scale production.
Alidep® Depth Filter has various media combinations, and is mainly composed of cellulose, filter aids, and positively charged resins. It provides strong dirt-holding capacity and may be used for the clarification of cell culture fluid.
-
-
-
Product Characteristics
Product Specifications
Product Series
Alidep® process development
scale (L series)
Alidep® laboratory scale
(P series)
Alidep® production scale
(M series)
Effective Filtration Area
23 cm2
270 cm2、540 cm2
0.11 m2、0.55 m2、1.1 m2
Overall Dimension
9.4x7.1x4.3 cm
22x14x(7.4~9.6) cm
62x32x(3.1~12.4) cm
Materials of
Construction
Inlet, Outlet and Exhaust Port
Luer connector:
Polypropylene
6 mm hose connector:
Glass fiber-filled polypropylene
Flat end connector:
Glass fiber-filled polypropylene
Frame and Cover Material
Glass fiber-filled polypropylene
Filter Plate
Cellulose, filter aids and resin
Supporting Layer
Polypropylene
O-ring
Silicone
Working characteristics
Aerosol Challenge Test
Aerosol leakage < 0.01%
Maximum Operating Pressure
3.5 bar @ 40°C
Maximum Forward Differential
Pressure
3.0 bar @ 25℃
Maximum Reverse Differential
Pressure
2.0 bar @ 25℃
Sterilization
Resistance
Autoclave: 125℃ 60 min,2cycles
Indirect Food Additive
All component materials meet the requirements of Indirect Food Additive cited in FDA 21 CFR 177-182
Toxicity
The raw materials of all Alidep® Depth Filter components, including filter plates ( mainly made from cellulose, filter aids and resin ), frames and covers made from glass fiber-filled polypropylene, depth supporting nets made from polypropylene, and silicone O-rings, meet the biological safety requirements refering to USP <88> Class VI plastic
Bacterial Endotoxin
The endotoxin level of rinsing liquid is <0.25 EU/mL, which complies with the requirements for water for injection in Chinese Pharmacopoeia and United States Pharmacopoeia
※The thickness of filter is related to the number of filter plate layers
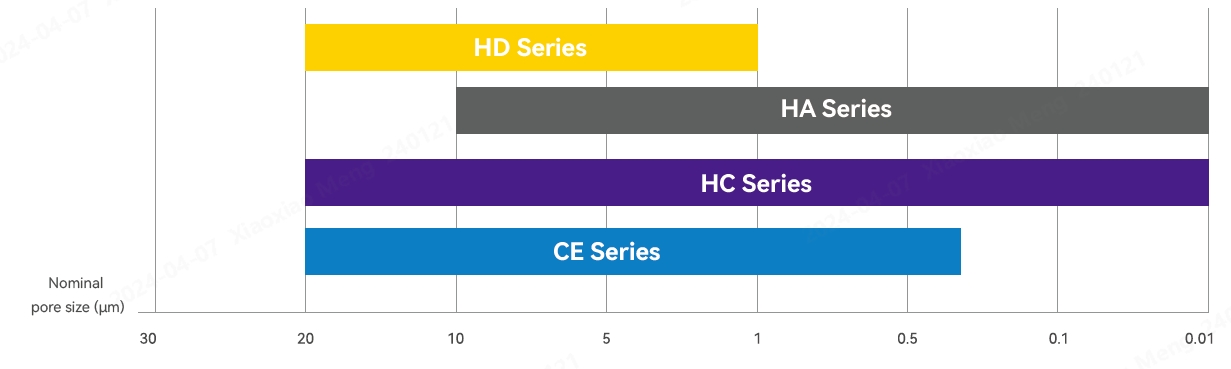
Pore Size Range
Name of medium
Application
Features
Composition
Double-layer HD Series
Primary clarification of cell culture fluid
Two-stage filter plate and open pore, which can retain large particles and fragments
Cellulose, filter aids and resin
Double-layer HA Series
Secondary clarification of cell culture fluid
Double-stage filter plate and dense structure, which can achieve more strict turbidity control
Cellulose, filter aids and resin
Double-layer HC Series
Direct clarification of cell culture fluid
Double-stage filter plate, which is designed to clarify material liquid with a low cell density
Cellulose, filter aids and resin
Double-layer CE Series
Clarification of plasmids and virus particles, etc.
Double-stage filter plate, which features low adsorption
Cellulose
Pore Size Range
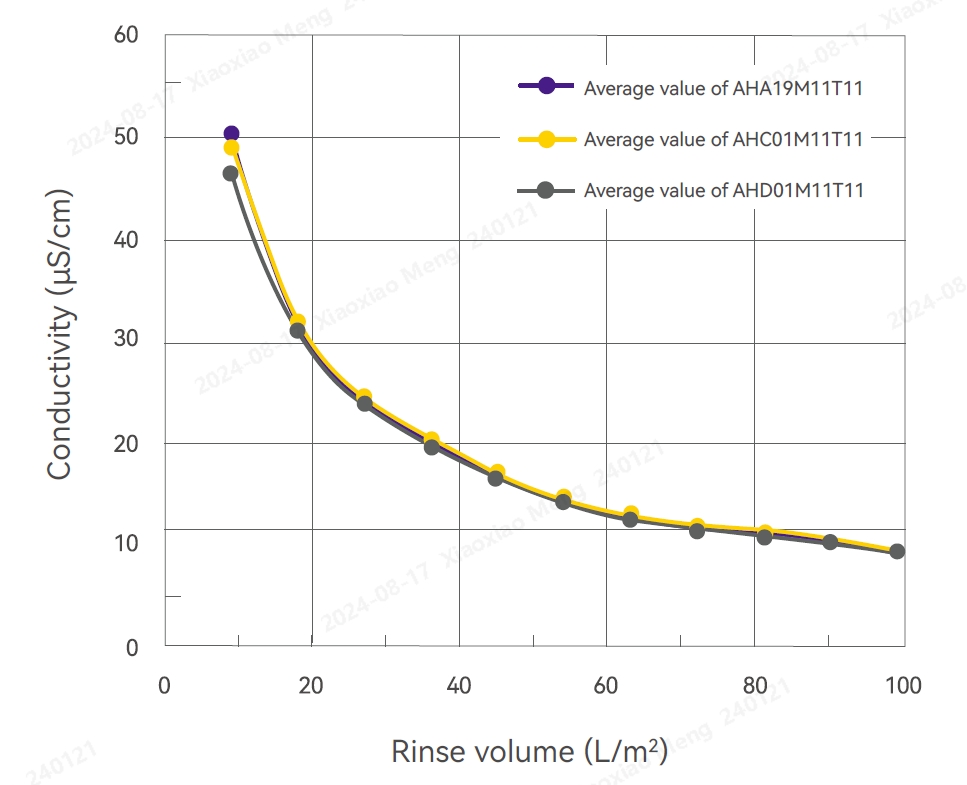
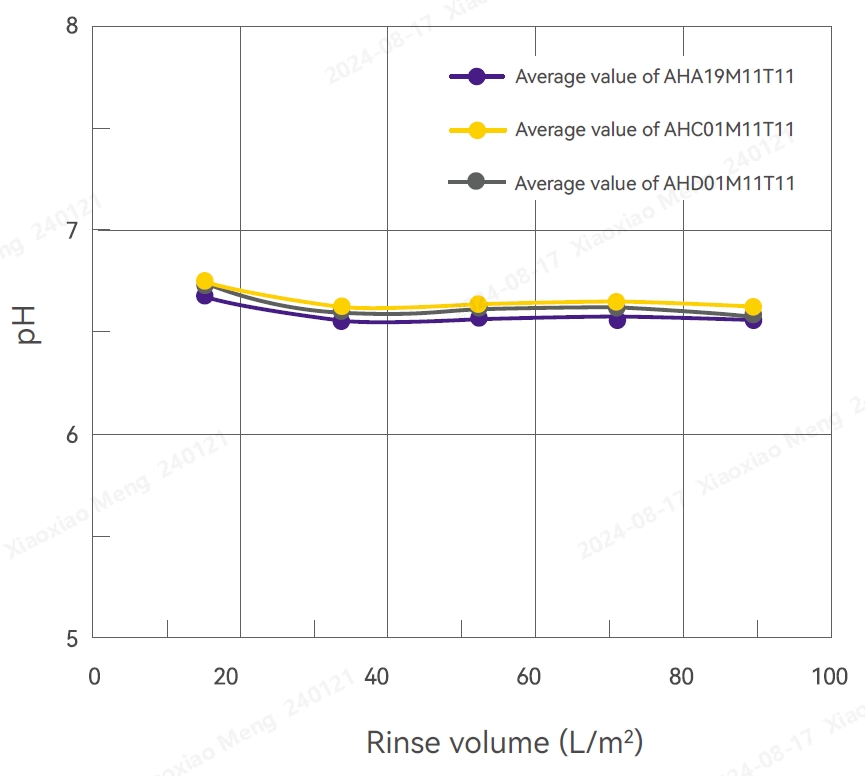
Conductivity Test Results pH Test Results
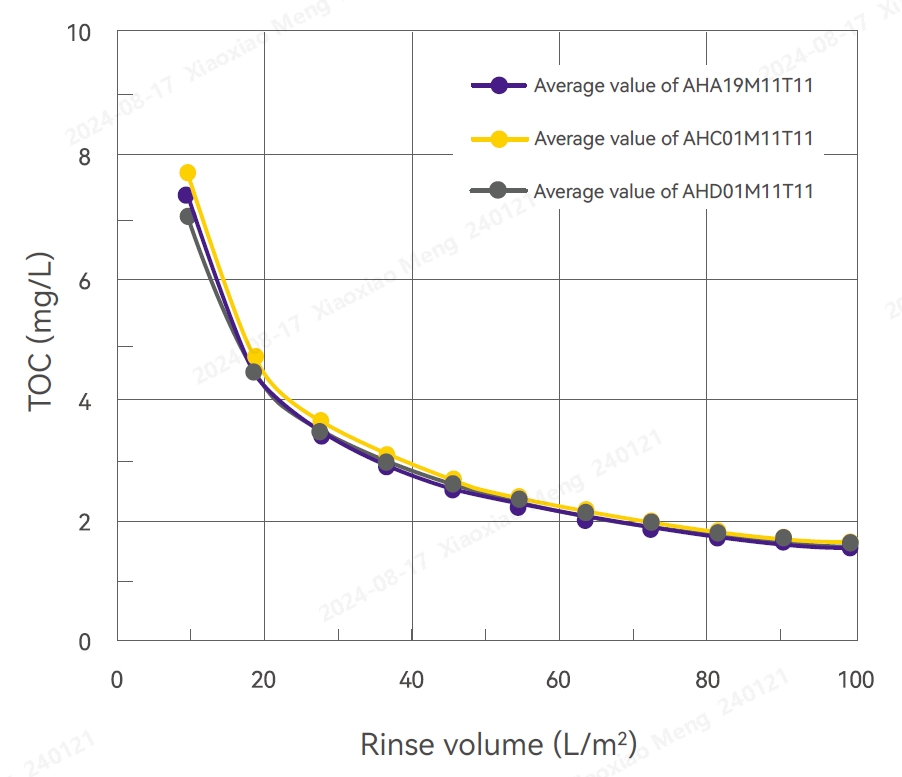
TOC Test Results Test Results of Flow Differential Pressure
Validation Methods and Results
Conductivity
Several filters were randomly selected from different production batches and subjected to high-pressure sterilization at 125°C for 60 minutes. These filters were then rinsed with 100 L/m2 pure water for 10-20 minutes. The conductivity ranged from 2.79 to 7.51 μS/cm
NVR
Several filters were randomly selected from different production batches and subjected to high-pressure sterilization at 125°C for 60 minutes. These filters were then rinsed with 100 L/m2 pure water for 10-20 minutes. The NVR ranged from 667 to 1,314 mg
TOC
Several filters were randomly selected from different production batches and subjected to high-pressure sterilization at 125°C for 60 minutes. These filters were then washed with 100 L/m2 pure water for 10-20 minutes. The TOC ranged from 1.12 to 1.55 mg/L
pH
Several filters were randomly selected from different production batches and subjected to high-pressure sterilization at 125°C for 60 minutes. These filters were then rinsed with 100 L/m2 pure water for 10-20 minutes. The pH ranged from 6.10 to 6.62
Endotoxin
Several filters were randomly selected from different production batches, then rinsed with 100L/m2 pure water for 10-20minutes, followed by soaking in pure water for 24 hours. The endotoxin level was <0.25 EU/mL as indicated by Tachypleus Amebocyte Lysate test
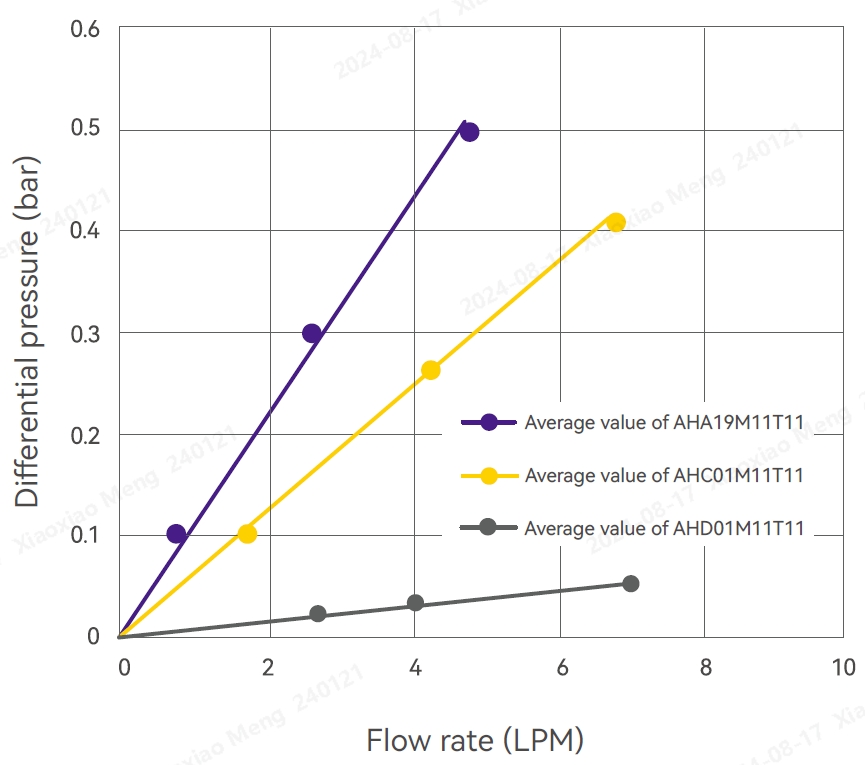
Flow differential pressure
Several filters were randomly selected from different production batches and subjected to high-pressure sterilization at 125°C for 60 minutes. These filters were then rinsed with 100 L/m2 pure water for 10-20 minutes. The experiment was conducted at room temperature. After venting, the flow rate was gradually increased, and the upstream and downstream pressures were observed. Within 8 LPM, the differential pressure was < 0.6 bar
Tolerated liquid pressure
Several filters were randomly selected from different production batches and subjected to high-pressure sterilization at 125°C for 60 minutes, twice. The tightness of filter was tested. High-viscosity solution was filtered to increase the upstream and downstream differential pressure of filter. The filter was subjected to forward and reverse pulse pressures at room temperature, followed by rinsing, drying and tightness challenge test. At room temperature, Alidep® Depth Filter can withstand 3.0 bar forward pressure and 2.0 bar reverse pressure








