Depth Stacked Filter
-
-
-
Product Specifications
Product Specifications
Product Series
16 inch 8 cells
16 inch 10 cells
16 inch other
Effective Filtration Area
1.8 m2
2.3 m2
Optional
Height
184 mm
275 mm
-
Materials of
Construction
Filter Media
Filter Media Cellulose, Filter Aid, Resin or Cellulose
Frame and Supporter
Glass Fiber Reinforced Polypropylene
Stainless Steel Band
316L
O-ring
Silicone
Working characteristics
Particle Challenge
Particle Intercept>90%
Max Operation Pressure
3.0 bar @ 25℃
Max Pressure Differentialal
Forward:2.0 bar @ 25℃
Reverse:not recommended
Sterilization
Resistance
In-line steam: 126℃ 30 min, 3 cycles
Indirect Food Additive
All component materials meet the requirements of Indirect Food Additive cited in FDA 21 CFR 177-182
Toxicity
The raw materials of all Alidep® Depth Stacked Filter components meet the biological safety requirements refering to USP<88> Class VI plastic
Bacterial Endotoxin
The endotoxin level of rinsing liquid is <0.25 EU/mL, which complies with the requirements for water for injection in Chinese Pharmacopoeia and United States Pharmacopoeia
Conductivity Test Results pH Test Results
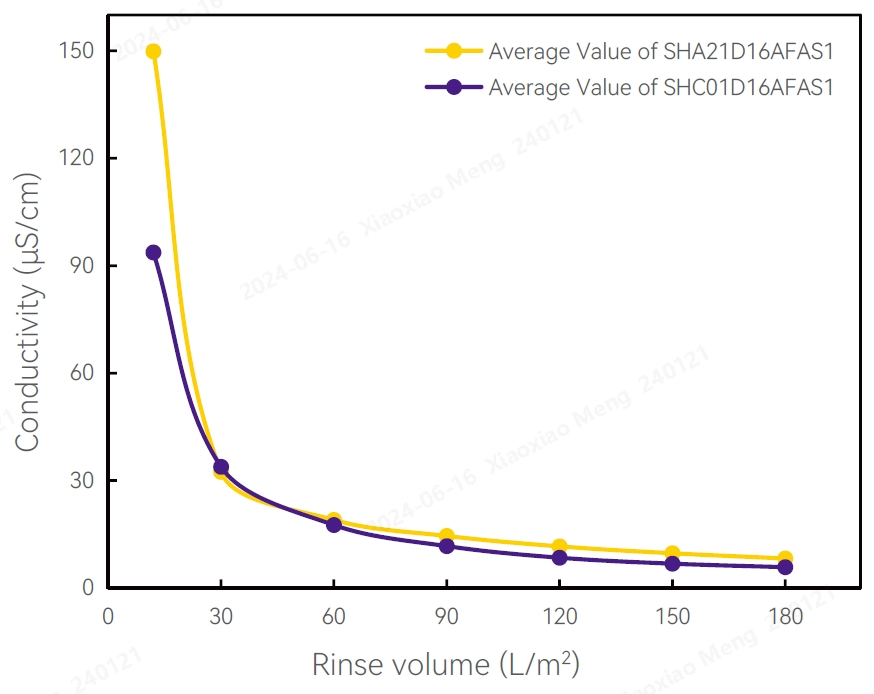
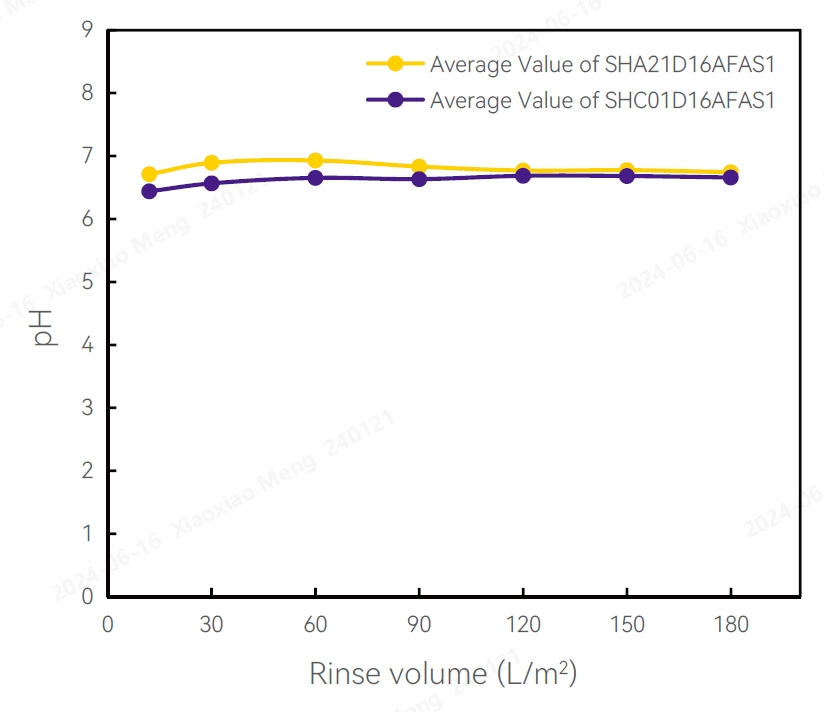
TOC Test Results Test Results of Flow Differential Pressure
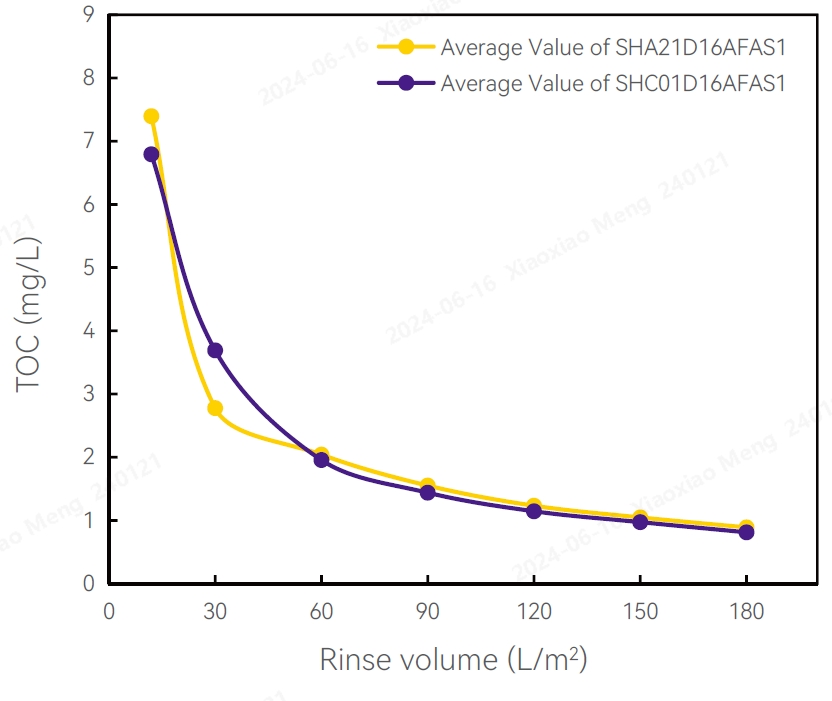
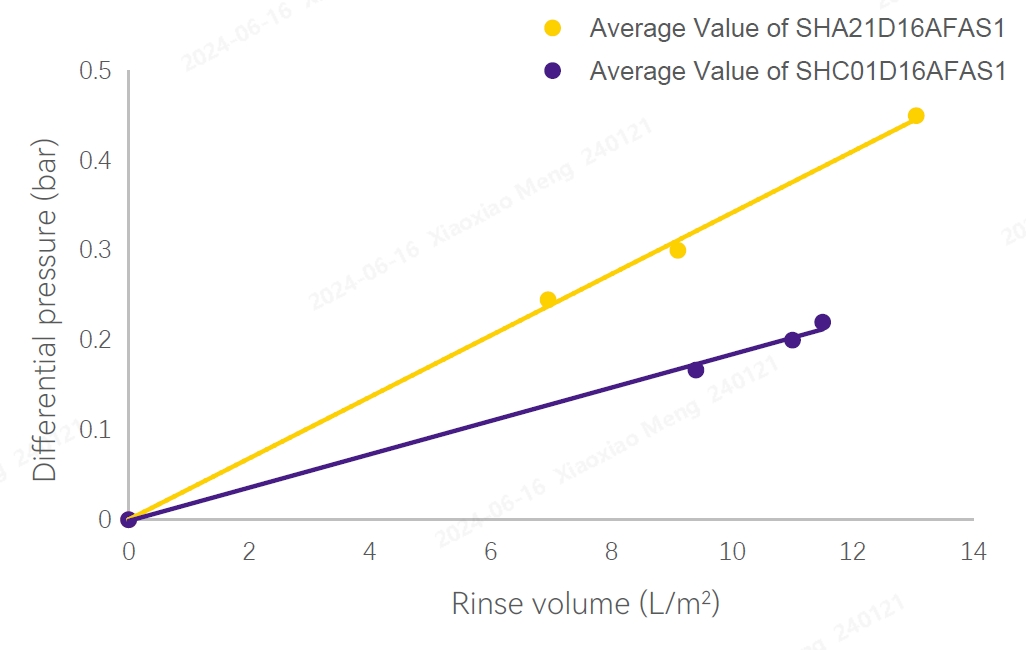
Validation Methods and Results
Conductivity
Several filters were randomly selected from different production batches, then rinsed with 100L/m2 pure water for 10-20 minutes. The conductivity ranged from 5-8 μS/cm.
TOC
Several filters were randomly selected from different production batches, then rinsed with 100L/m2 pure water for 10-20 minutes. The TOC was about 1 mg/L.
pH
Several filters were randomly selected from different production batches, then rinsed with 100L/m2 pure water for 10-20 minutes. The pH ranged from 6.6-6.8.
Endotoxin
Several filters were randomly selected from different production batches, then rinsed with 100L/m2 pure water for 10-20 minutes, followed by soaking in pure water for 24 hours. The endotoxin level was < 0.25 EU/mL as indicated by Tachypleus Amebocyte Lysate test.
Endurance to Sterilization
Several filters were randomly selected from different production batches, after 3 cycles of SIP at 126℃ for 30min, filters passed air tightness, diffusion flow, hydraulic stress. Flow rate and pressure dropafter SIP met the product quality standard.
Hydraulic Stress
Several filters were randomly selected from different production batches, respectively conduct hydraulic stress test at ambient temperature and after 3 cycles of SIP at 123℃ for 30 min. High-viscosity solutions was filtered to increase the pressure drop across the filters. Apply forward pulse pressure to the filter at ambient temperature, rinseand conduct diffusion flow. Depth stacked filter can withstand 2.0bar forward pressure at ambient temperature.



