CHC Sterilizing-Grade PES Capsule Filter
Alioth Alipore® CHC double-layer polyethersulfone (PES) filters are suitable for a variety of sterile filtration of process fluids with both cartridge and disposable capsule format available. The filter has passed strict sterilization performance validation. It features a built-in pre-filtration layer to achieve high flow rate and capacity, and thereby optimize your process efficiency and cost effectiveness.
-
-
-
Product Specifications—Alicap® L-type Capsule Filter
Product Specifications
Alicap® L300
Alicap® L600
Alicap® L02
Alicap® L04
Alicap® L5
Alicap® L10
Alicap® L20
Body Diameter
Maximum Width
Maximum Length67 mm
77 mm
100.5 mm67 mm
77 mm
122 mm72 mm
96 mm
164 mm72 mm
96 mm
217 mm107 mm
147 mm
224 mm107 mm
147 mm
362 mm92 mm
132 mm
575 mm
Materials of
Construction
Membrane
Hydrophilic polyethersulfone (PES)
Supporting Layer
Polypropylene (PP)
O-rings
Silicone
Core/Cage/End caps/Housing
Polypropylene (PP)
Integrity testing
Bubble Point
≥3520 mbar(51 psi)(wetted with H2O, 20℃, compressed air)
Diffusion Flow
Test pressure 2760 mbar (40 psi) (20℃, compressed air )
-
≤2.5 mL/min
≤5.5 mL/min
≤9.5 mL/min
≤12.5 mL/min
≤25 mL/min
≤50 mL/min
Working characteristics
Maximum Differential
Pressure
Forward: 5.0 bar(72.5 psi)@ 25℃/3.0 bar(43.5 psi)@ 80℃
Reverse: 2.0 bar(29.0 psi)@ 25℃
Sterilization
Resistance
Autoclave(A&G): 131℃, 30 min, 3 cycles
Gamma-compatible (G): 25-45 kGy
*Capsule filters can't be sterilized by SIP,
sterile type capsule filters can't be sterilized again
Autoclave(A&G): 131℃, 30 min, 5 cycles
Gamma-compatible (G): 25-45 kGy
*Capsule filters can't be sterilized by SIP,
sterile type capsule filters can't be sterilized again
Filtration Area
230 cm2
430 cm2
0.11 m2
0.19 m2
0.3 m2
0.6 m2
1.2 m2
Pore Size
0.45+0.2 μm
Bacterial Retention
>107 cfu/cm2 B. diminuta ( ATCC® 19146TM )
Biological Safety
All construction components meet the biological safety requirements refering to USP<88> Class VI plastic and USP<87>
Indirect Food Additive
All component materials meet the requirements of Indirect Food Additive cited in EU 1935/2004/EC and FDA 21 CFR 177-182
Cleanliness
Meets the requirements for a ‘non-fiber releasing’ filter defined in FDA 21 CFR 211.72 and 210.3 (b) (5) (6), and particulate matter meets the requirements of USP<788>
Endotoxin
Endotoxin﹤0.25 EU/mL by LAL test method, which complies with the requirements of USP<85>
TOC/Conductivity
TOC﹤0.5 mg/L, Conductivity﹤1.3 μS/cm
Manufacturing
Integrity
100% integrity test passed
Manufacturing
Environment
Manufactured in conformance with cGMP
Typical Flow Characteristics
Water Flow Rate and Pressure Drop-Alipore® CHC 0.45+0.2μm Alicap L-type Capsule Filter
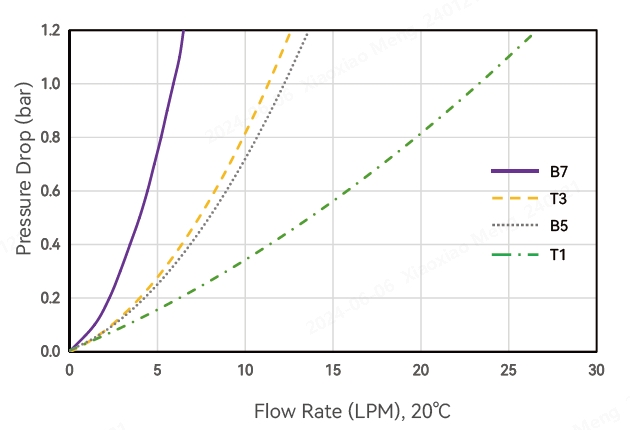
*T1, T3, B5, B7 represent different interface types, see order information for details.



