Single-layer Cartridge Filter (PES 0.2μm)
Product Introduction
Alioth Alipore® DHF Single-layer Cartridge Filter is a 0.2μm single-layer polyethersulfone (PES) filter that can efficiently retain bacteria (LRV > 6) and particulate contaminants. It has wide chemical compatibility and low leaching level, thus suitable for the bioburden control and pre-filtration of various fluids. It can provide more effective protection at different stages of bioprocess, prolonging the service life of sterilizing filters and safeguarding other processing systems. This filter is produced in a controlled environment, and its manufacturing process conforms to ISO9001 quality system standards. Each cartridge filter undergoes an integrity testing during the process of manufacturing.
Key Features and Benefits
- High flow rate, high capacity
- Verified bioburden control capability
- Withstand multiple cycles of sterilization
- Wide chemical compatibility
Typical Applications
- Bioburden control
- Filtration of terminally sterilized products
- Filtration of clarified cell culture fluid
- Pre-filtration for column protection
- Intermediate product filtration
- Pre-filtration before terminal sterilization, etc.
Typical Flow Characteristics
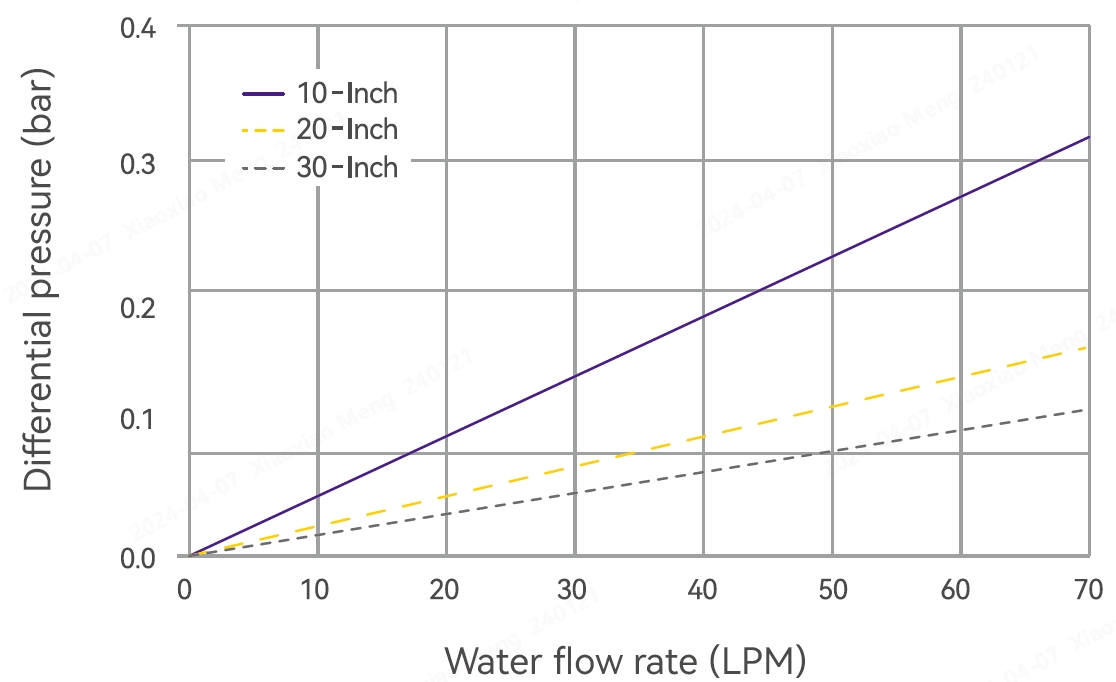
Product Specifications
| 10 inch | 20 inch | 30 inch | |
|
Dimensions Diameter |
69 mm |
69 mm |
69 mm |
| Filtration Area | 0.66 m2 | 1.32 m2 | 1.98 m2 |
| Pore size | 0.2 μm | ||
| Materials of Construction |
Filter membrane: Hydrophilic polyethersulfone (PES) Supporting Layer: Polypropylene (PP) Liner/Cartridge body: Polypropylene (PP) |
||
| Bacterial Retention | > 106 / 10inch B. diminuta ( ATCC® 19146™ ) | ||
| Maximum Differential Pressure | Forward: 5 bar ( 72.5 psi ) @ 25℃ / 1.5 bar ( 21.75 psi ) @ 80℃ / Reverse: 2 bar ( 29 psi ) @ 25℃ | ||
| Bubble Point | ≥3180 mbar(51 psi)(wetted with H₂O, 20℃, compressed air) | ||
| Sterilization Parameters | 135℃, 30 minutes, 20 cycles of high-pressure sterilization or 10 cycles of sterilization in place | ||
| Biological Safety | All the construction components of this cartridge comply with the requirements for biological safety of Class Vl plastics in current USP < 88 > | ||
| Indirect Food Additive | The primary construction components of this cartridge comply with the reguirements for food contact materials as stipulated in EU 1935 / 2004 / EC and FDA 21 CFR 177-182 | ||
| Cleanliness | Cleanliness meets the requirements for nonfiber releasing filter as specified in FDA 21 CFR 211.72 and 210.3 (b) (5) (6), and the level of insoluble particles in rinsing liquid meets the requirements of USP < 788 > | ||
| Endotoxin | The endotoxin level of cartridge rinsing liquid is < 0.25 EU/mL as indicated by gel method, which complies with the requirements of USP < 85 > | ||
| Integrity | Each filter has passed the integrity test | ||
| TOC / Conductivity | TOC < 0.5 mg/L, Conductivity < 1.3 μS/cm | ||
| Manufacturing Environment | Manufactured in conformance with cGMP | ||
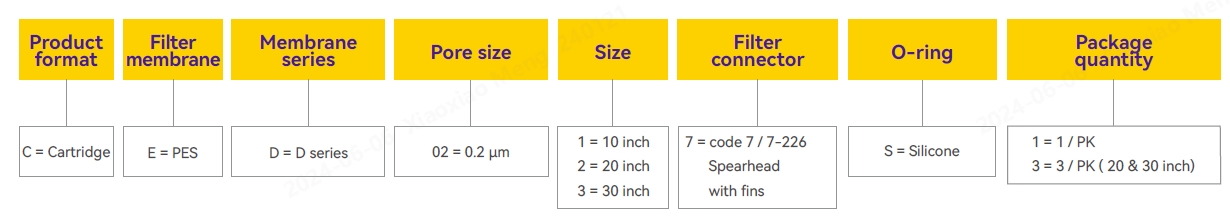
Ordering Information
For further information, please contact us and Alioth technical team will provide a comprehensive service for your filtration process.


