CHC Sterilizing-Grade PES Capsule Filter
Product Specifications—Alicap® L-type Capsule Filter
|
Product Specifications |
|
Alicap® L300 |
Alicap® L600 |
Alicap® L02 |
Alicap® L04 |
Alicap® L5 |
Alicap® L10 |
Alicap® L20 |
|
Body Diameter |
67 mm |
67 mm |
72 mm |
72 mm |
107 mm |
107 mm |
92 mm 132 mm 575 mm |
|
|
Materials of Construction |
Membrane |
Hydrophilic polyethersulfone (PES) |
||||||
|
Supporting Layer |
Polypropylene (PP) |
|||||||
|
O-rings |
Silicone |
|||||||
|
Core/Cage/End caps/Housing |
Polypropylene (PP) |
|||||||
|
Integrity testing |
Bubble Point |
≥3520 mbar(51 psi)(wetted with H2O, 20℃, compressed air) |
||||||
|
Diffusion Flow |
Test pressure 2760 mbar (40 psi) (20℃, compressed air ) |
|||||||
|
- |
≤2.5 mL/min |
≤5.5 mL/min |
≤9.5 mL/min |
≤12.5 mL/min |
≤25 mL/min |
≤50 mL/min |
||
|
Working characteristics |
Maximum Differential Pressure |
Forward: 5.0 bar(72.5 psi)@ 25℃/3.0 bar(43.5 psi)@ 80℃ Reverse: 2.0 bar(29.0 psi)@ 25℃ |
||||||
|
Sterilization Resistance |
Autoclave(A&G): 131℃, 30 min, 3 cycles Gamma-compatible (G): 25-45 kGy *Capsule filters can't be sterilized by SIP, sterile type capsule filters can't be sterilized again |
Autoclave(A&G): 131℃, 30 min, 5 cycles Gamma-compatible (G): 25-45 kGy *Capsule filters can't be sterilized by SIP, sterile type capsule filters can't be sterilized again |
||||||
|
Filtration Area |
230 cm2 |
430 cm2 |
0.11 m2 |
0.19 m2 |
0.3 m2 |
0.6 m2 |
1.2 m2 |
|
|
Pore Size |
0.45+0.2 μm |
|||||||
|
Bacterial Retention |
>107 cfu/cm2 B. diminuta ( ATCC® 19146TM ) |
|||||||
|
Biological Safety |
All construction components meet the biological safety requirements refering to USP<88> Class VI plastic and USP<87> |
|||||||
|
Indirect Food Additive |
All component materials meet the requirements of Indirect Food Additive cited in EU 1935/2004/EC and FDA 21 CFR 177-182 |
|||||||
|
Cleanliness |
Meets the requirements for a ‘non-fiber releasing’ filter defined in FDA 21 CFR 211.72 and 210.3 (b) (5) (6), and particulate matter meets the requirements of USP<788> |
|||||||
|
Endotoxin |
Endotoxin﹤0.25 EU/mL by LAL test method, which complies with the requirements of USP<85> |
|||||||
|
TOC/Conductivity |
TOC﹤0.5 mg/L, Conductivity﹤1.3 μS/cm |
|||||||
|
Manufacturing Integrity |
100% integrity test passed |
|||||||
|
Manufacturing Environment |
Manufactured in conformance with cGMP |
|||||||
Typical Flow Characteristics
Water Flow Rate and Pressure Drop-Alipore® CHC 0.45+0.2μm Alicap L-type Capsule Filter
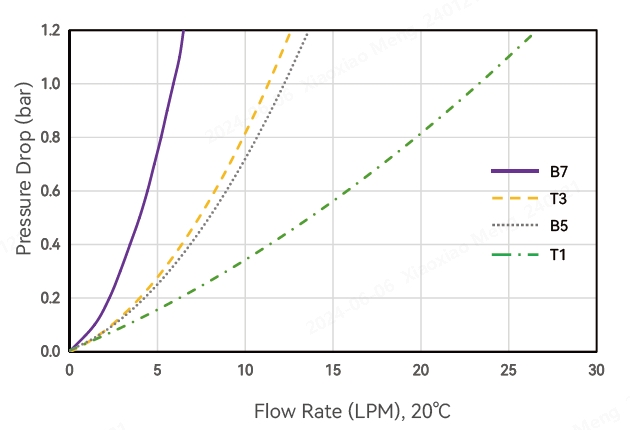
*T1, T3, B5, B7 represent different interface types, see order information for details.


